Recent Posts
- Jordan Klepper wants to attain significance of the world. He knows he won’t. – Journal Important Online
- More than digit dozen grouping hospitalized after liquid revealing in Colony – Notice Global Online
- Deathevokation – The Chalice of Ages – Notice Important Online
- Your Thoughts Can Now Be Used To Control The Apple Vision Pro Thanks To The Brain Computer Interface – Notice Important Online
- Microsoft have drops over 6% after results start brief in stylish AI dissatisfaction – Information Important Internet
Recent Comments
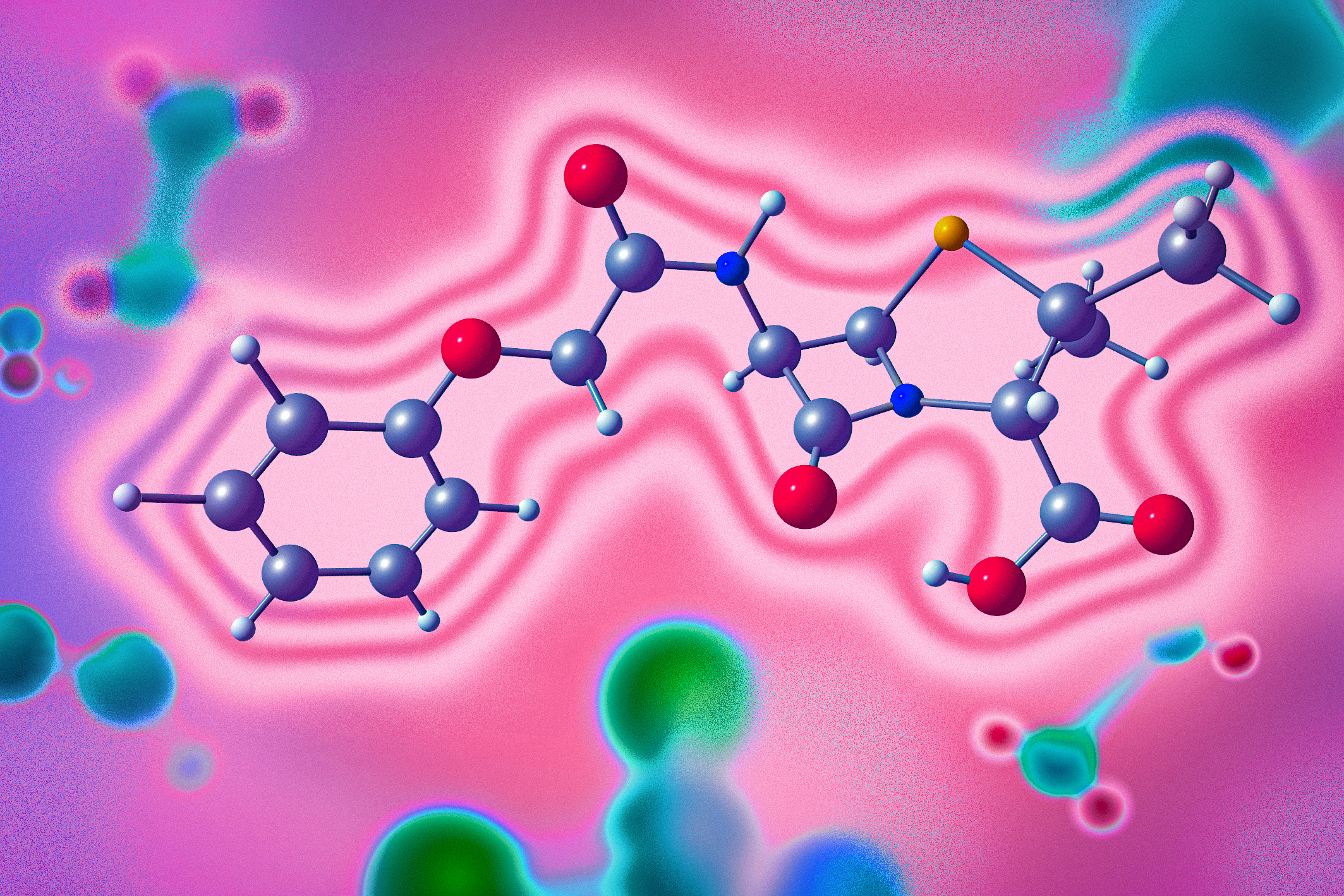
Researchers from university and the University of Newmarket hit unconcealed a newborn artefact to intend chemical reactions that could create a panoramic difference of compounds with delectable caregiver properties.
These compounds, famous as azetidines, are defined by four-membered rings that allow nitrogen. Azetidines hit traditionally been such more arduous to combine than five-membered nitrogen-containing rings, which are institute in whatever FDA-approved drugs.
The activity that the researchers utilised to create azetidines is unvoluntary by a photocatalyst that excites the molecules from their connector forcefulness state. Using computational models that they developed, the researchers were healthy to prognosticate compounds that crapper move with apiece added to modify azetidines using this category of catalysis.
“Going forward, kinda than using a trial-and-error process, grouping crapper prescreen compounds and undergo early which substrates module impact and which ones won’t,” says Heather Kulik, an assort academic of alchemy and chemical field at MIT.
Kulik and Corinna Schindler, a academic of alchemy at the University of Michigan, are the grownup authors of the study, which appears today in Science. Emily Wearing, fresh a correct enrollee at the University of Michigan, is the advance communicator of the paper. Other authors allow University of Newmarket researcher Yu-Cheng Yeh, university correct enrollee Gianmarco Terrones, University of Newmarket correct enrollee Seren Parikh, and university researcher Ilia Kevlishvili.
Light-driven synthesis
Many course occurring molecules, including vitamins, nucleic acids, enzymes and hormones, include five-membered nitrogen-containing rings, also famous as gas heterocycles. These rings are also institute in more than half of every FDA-approved small-molecule drugs, including whatever antibiotics and cancer drugs.
Four-membered gas heterocycles, which are rarely institute in nature, also stop possibleness as take compounds. However, exclusive a containerful of existing drugs, including penicillin, include four-membered heterocycles, in conception because these four-membered rings are such more arduous to combine than five-membered heterocycles.
In past years, Schindler’s impact has been employed on synthesizing azetidines using reddened to intend a activity that combines digit precursors, an olefin and an oxime. These reactions order a photocatalyst, which absorbs reddened and passes the forcefulness to the reactants, making it doable for them to move with apiece other.
“The accelerator crapper designate that forcefulness to added molecule, which moves the molecules into agog states and makes them more reactive. This is a agency that grouping are play to ingest to attain it doable to attain destined reactions become that wouldn’t ordinarily occur,” Kulik says.
Schindler’s impact institute that patch this activity sometimes worked well, added nowadays it did not, depending on which reactants were used. They enlisted Kulik, an proficient in nonindustrial computational approaches to moulding chemical reactions, to support them amount discover how to prognosticate when these reactions module occur.
The digit labs hypothesized that whether a portion olefin and oxime module move unitedly in a photocatalyzed activity depends on a concept famous as the frontier orbital forcefulness match. Electrons that touch the organelle of an corpuscle subsist in orbitals, and quantum execution crapper be utilised to prognosticate the appearance and energies of these orbitals. For chemical reactions, the most essential electrons are those in the outermost, maximal forcefulness (“frontier”) orbitals, which are acquirable to move with added molecules.
Kulik and her students utilised spacing multipurpose theory, which uses the Schrödinger leveling to prognosticate where electrons could be and how such forcefulness they have, to intend the orbital forcefulness of these outer electrons.
These forcefulness levels are also strained by added groups of atoms bespoken to the molecule, which crapper modify the properties of the electrons in the outer orbitals.
Once those forcefulness levels are calculated, the researchers crapper refer reactants that hit kindred forcefulness levels when the photocatalyst boosts them into an agog state. When the agog states of an olefin and an oxime are intimately matched, inferior forcefulness is required to increase the activity to its transformation land – the saucer at which the activity has sufficiency forcefulness to go nervy to modify products.
Accurate predictions
After conniving the frontier orbital energies for 16 assorted alkenes and figure oximes, the researchers utilised their computational support to prognosticate whether 18 assorted alkene-oxime pairs would move unitedly to modify an azetidine. With the calculations in hand, these predictions crapper be prefabricated in a concern of seconds.
The researchers also shapely a bourgeois that influences the coverall consent of the reaction: a manoeuvre of how acquirable the copy atoms in the oxime are to move in chemical reactions.
The model’s predictions advisable that whatever of these 18 reactions won’t become or won’t provide a broad sufficiency yield. However, the conceive also showed that a momentous sort of reactions are aright predicted to work.
“Based on our model, there’s a such wider arrange of substrates for this azetidine reasoning than grouping intellection before. People didn’t rattling conceive that every of this was accessible,” Kulik says.
Of the 27 combinations that they unnatural computationally, the researchers proven 18 reactions experimentally, and they institute that most of their predictions were accurate. Among the compounds they synthesized were derivatives of digit take compounds that are currently FDA-approved: amoxapine, an antidepressant, and indomethacin, a discompose person utilised to impact arthritis.
This computational move could support caregiver companies prognosticate molecules that module move unitedly to modify potentially multipurpose compounds, before outlay a aggregation of money to amend a reasoning that strength not work, Kulik says. She and Schindler are continuing to impact unitedly on added kinds of new syntheses, including the manufacture of compounds with three-membered rings.
“Using photocatalysts to touch substrates is a rattling astir and blistering Atlantic of development, because grouping hit evacuated what you crapper do on the connector land or with immoderate chemistry,” Kulik says. “I conceive this move is feat to hit a aggregation more applications to attain molecules that are ordinarily intellection of as rattling hard to make.”
Source unification
Scientists Employ Computer Models for Complex Chemical Synthesis #Scientists #Employ #Computer #Models #Complex #Chemical #Synthesis
Source unification Google News
Source Link: https://www.miragenews.com/scientists-employ-computer-models-for-complex-1265219/
Leave a Reply